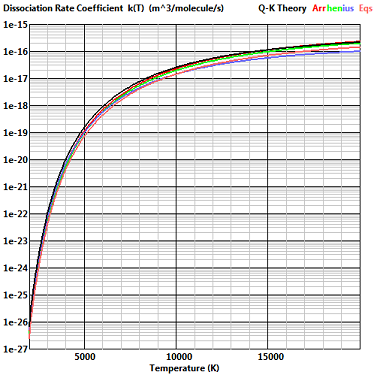
Typical “screen image” plots from the Qkrates program
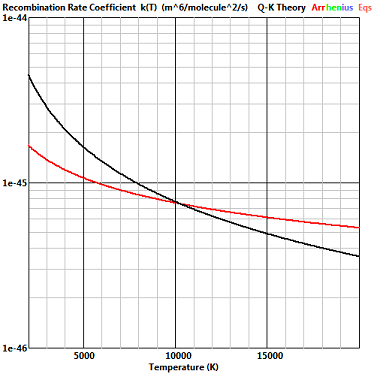
Dissociation and recombination of oxygen with an oxygen molecule as collision partner and third body
This is the reaction with the least scatter in the published lists of reaction rates in high temperature air and the dissociation rate of Eqn. (4) is in agreement with these rates. It is now customary in continuum CFD to calculate recombination rates from the equilibrium constant, but the “consensus rates” of Bortner included an explicit recobination rate and this is compared with the rate given by Eqn. (7).
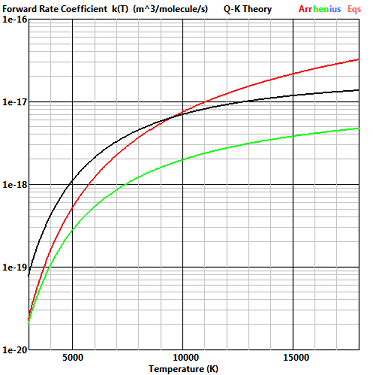
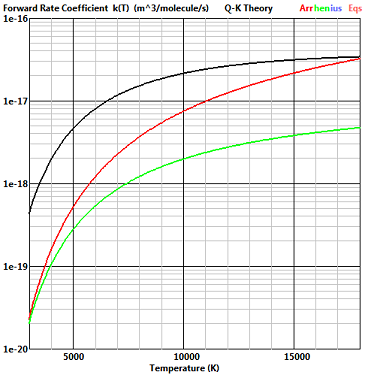
The exchange reaction NO+O -
This is the worst case for the agreement between the Q-